Findings may lead to new tissue cryopreservation methods for grafts and organ transplantations
November 6, 2013
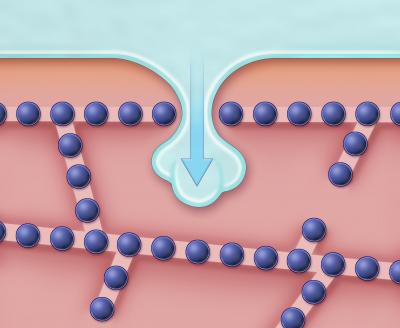
This
illustration shows how ice invades the space between two cells, an
event that triggers crystallization of the cell water. The view shown
here is a cross-section through the interface where the two cells meet.
“Tight junctions,” which are depicted here as small spheres arrangesd in
rows, are embedded in the cell membrane and form seams that stitch the
two cells together. Although these seams normally act as barriers that
prevent ice from invading the tissue, the research by Higgins and
Karlsson demonstrated that if the temperature is sufficiently low,
extracellular ice can penetrate through nanoscale openings in the tight
junction seams. As a result, the cells in the vicinity of the breach
become much more susceptible to freezing damage. (Credit: Scott
Leighton)
Developing an efficient way to freeze and store living tissues could transform many aspects of medical care and research, but ice crystallization often occurs within cells during such cryopreservation procedures, leading to cell death.
A long-standing obstacle to avoiding tissue damage during freezing is that when cells are joined together within tissues, individual cells are more likely to crystallize than if the cells are kept apart.
“In tissues, ice crystals are thought to be able to grow through membrane channels called gap junctions, thus allowing ice to easily propagate from cell to cell,” explains senior author Dr. Jens Karlsson, of the Department of Mechanical Engineering at Villanova University.
“But the results of the present study indicate that the mechanism of tissue cryo-injury is much more complex than was previously thought.”
Dr. Karlsson and his team monitored microscopic freezing events inside genetically modified cells and used mathematical models to show that gap junctions do not always provide the major pathway for the spreading of ice crystals between cells. They saw that cell-to-cell propagation of ice also occurred during freezing of tissue samples in which gap junction formation had been suppressed.
The authors discovered that intercellular connections — in which neighboring cell membranes are stitched together by rows of rivet-like structures known as tight junctions — also play a significant role.
“By using high-speed video imaging, we found evidence that ice from outside the cells sometimes forms nanoscale branches, which can penetrate the barriers created by tight junction seams,” says Dr. Karlsson. “The resulting invasion of the spaces between cells appears to promote crystallization of the cells adjacent to the breach.”
The unexpected findings may provide a boon for the manufacturing of engineered tissue products that can be used for grafts and organ transplantations. “Using cryopreservation to stop living tissue constructs from spoiling during storage will be the key to enabling economical mass production, quality assurance, and shipping logistics for these life-saving products,” says Dr. Karlsson.
These findings could also conceivably lead to improved cryopreservation technology for cryonics (preserving human life in a state that will be viable and treatable by future medicine). — Editor
Abstract of Biophysical Journal paper
The development of cryopreservation procedures for tissues has proven to be difficult in part because cells within tissue are more susceptible to intracellular ice formation (IIF) than are isolated cells. In particular, previous studies suggest that cell-cell interactions increase the likelihood of IIF by enabling propagation of ice between neighboring cells, a process thought to be mediated by gap junction channels. In this study, we investigated the effects of cell-cell interactions on IIF using three genetically modified strains of the mouse insulinoma cell line MIN6, each of which expressed key intercellular junction proteins (connexin-36, E-cadherin, and occludin) at different levels. High-speed video cryomicroscopy was used to visualize the freezing process in pairs of adherent cells, revealing that the initial IIF event in a given cell pair was correlated with a hitherto unrecognized precursor phenomenon: penetration of extracellular ice into paracellular spaces at the cell-cell interface. Such paracellular ice penetration occurred in the majority of cell pairs observed, and typically preceded and colocalized with the IIF initiation events. Paracellular ice penetration was generally not observed at temperatures >−5.65°C, which is consistent with a penetration mechanism via defects in tight-junction barriers at the cell-cell interface. Although the maximum temperature of paracellular penetration was similar for all four cell strains, genetically modified cells exhibited a significantly higher frequency of ice penetration and a higher mean IIF temperature than did wild-type cells. A four-state Markov chain model was used to quantify the rate constants of the paracellular ice penetration process, the penetration-associated IIF initiation process, and the intercellular ice propagation process. In the initial stages of freezing (>−15°C), junction protein expression appeared to only have a modest effect on the kinetics of propagative IIF, and even cell strains lacking the gap junction protein connexin-36 exhibited nonnegligible ice propagation rates.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington