A durable, low-cost water splitter made of silicon and nickel
New fuel cells can more efficiently generate electricity when the sun isn't shining or demand is high
November 18, 2013
[+]
Stanford
researchers have developed an inexpensive, corrosion-free device that
uses light to split water into oxygen and clean-burning hydrogen.
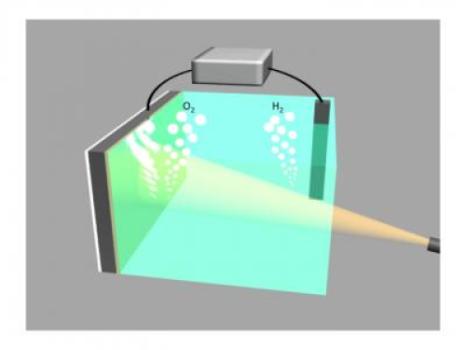
This
image shows two electrodes connected via an external voltage source
splitting water into oxygen (O2) and hydrogen (H2). The illuminated
silicon electrode (left) uses light energy to assist in the
water-splitting process and is protected from the surrounding
electrolyte by a 2-nm film of nickel. (Credit: Guosong Hong/Stanford
University)
The goal is to supplement solar cells with hydrogen-powered fuel cells that can generate electricity when the sun isn’t shining or demand is high.
The novel device — a silicon semiconductor coated in an ultrathin layer of nickel — could help pave the way for large-scale production of clean hydrogen fuel from sunlight, according to the scientists. Their results are published in the Nov. 15 issue of the journal Science.
“Solar cells only work when the sun is shining,” said study co-author Hongjie Dai, a professor of chemistry at Stanford. “When there’s no sunlight, utilities often have to rely on electricity from conventional power plants that run on coal or natural gas.”
A greener solution, Dai explained, is to supplement the solar cells with hydrogen-powered fuel cells that generate electricity at night or when demand is especially high.
Water splitting
To produce clean hydrogen for fuel cells, scientists have turned to an emerging technology called water splitting. Two semiconducting electrodes are connected and placed in water. The electrodes absorb light and use the energy to split the water into its basic components, oxygen and hydrogen. The oxygen is released into the atmosphere, and the hydrogen is stored as fuel.
When energy is needed, the process is reversed. The stored hydrogen and atmospheric oxygen are combined in a fuel cell to generate electricity and pure water. The entire process is sustainable and emits no greenhouse gases.
Nickel nanofilm
Finding a cheap, practical way to split water has been a major challenge. Existing solutions for water splitters, such as silicon electrodes coated with ultrathin layers of titanium dioxide and iridium, only work for hours and are expensive.
So the Stanford researchers turned to coating silicon electrodes with ordinary nickel. “Nickel is corrosion-resistant,” Stanford graduate student Michael J. Kenney, co-lead author of the Science study, said. “It’s also an active oxygen-producing catalyst, and it’s earth-abundant. That makes it very attractive for this type of application.”
For the experiment, the Dai team applied a 2-nanometer-thick layer of nickel onto a silicon electrode, paired it with another electrode and placed both in a solution of water and potassium borate. When light and electricity were applied, the electrodes began splitting the water into oxygen and hydrogen, a process that continued for about 24 hours with no sign of corrosion.
To further improve performance, the researchers experimented with adding lithium to the water-based solution. “Remarkably, adding lithium imparted superior stability to the electrodes,” Kenney said. “They generated hydrogen and oxygen continuously for 80 hours — more than three days — with no sign of surface corrosion.”
“Our lab has produced one of the longest-lasting silicon-based photoanodes,” Dai said. “The results suggest that an ultrathin nickel coating not only suppresses corrosion but also serves as an electrocatalyst to expedite the otherwise sluggish water-splitting reaction.
“Interestingly, a lithium addition to electrolytes has been used to make better nickel batteries since the Thomas Edison days. Many years later, we are excited to find that it also helps to make better water-splitting devices.”
“There have been many photoanode materials studied for water splitting over the past 40 years and the vast majority of them are metal oxides,” Kenney explained to KurzweilAI. “Oxide semiconductors are cheap and very stable but unfortunately split water at a rate that is currently too slow to be practical. Silicon-based photoanodes are able to split water at a much higher rate but are typically considered too fragile for practical use. Our electrode is able to split water at a high rate and is stable.”
However, he cautioned that “cheap and efficient water splitting using two semiconductors immersed in solution is very difficult to achieve and is still a long way from being commercially viable. Many more science and engineering advances need to be made before a system like this can be put on your roof.”
The scientists plan to do additional work on improving the stability and durability of nickel-treated electrodes of silicon as well as other materials.
Support was provided by the Precourt Institute for Energy and the Global Climate and Energy Project at Stanford and the National Science Foundation.
Abstract of Science paper
Silicon’s sensitivity to corrosion has hindered its use in photoanode applications. We found that deposition of a ~2-nanometer nickel film on n-type silicon (n-Si) with its native oxide affords a high-performance metal-insulator-semiconductor photoanode for photoelectrochemical (PEC) water oxidation in both aqueous potassium hydroxide (KOH, pH = 14) and aqueous borate buffer (pH = 9.5) solutions. The Ni film acted as a surface protection layer against corrosion and as a nonprecious metal electrocatalyst for oxygen evolution. In 1 M aqueous KOH, the Ni/n-Si photoanodes exhibited high PEC activity with a low onset potential (~1.07 volts versus reversible hydrogen electrode), high photocurrent density, and durability. The electrode showed no sign of decay after ~80 hours of continuous PEC water oxidation in a mixed lithium borate-potassium borate electrolyte. The high photovoltage was attributed to a high built-in potential in a metal-insulator-semiconductor-like device with an ultrathin, incomplete screening Ni/NiOx layer from the electrolyte.
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington