Laser slicing technique lets scientists explore the 3D structure of small objects
Awesome videos alert
October 15, 2013
[+]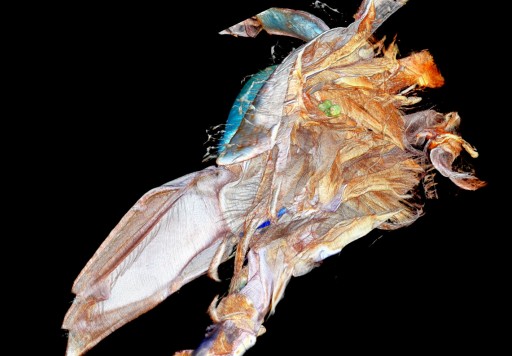
Penn State scientists have developed a powerful new tool for exploring the three-dimensional structure of small objects.
A bisected yellow jacket head (credit: Benjamin Hall/Penn State Materials Research Institute)
Using a nanosecond-pulse laser, then-student Benjamin Hall developed a method* to slice 11 identically spaced root samples per second.
“Then I had to take the samples all the way across campus to the root lab to have them analyzed,” Hall says.
“It was easier to buy a good camera lens and take the photos myself and send the files to the lab.”
Visualizing samples
[+]
By placing the root on a moveable platform beneath the laser, Hall
could incrementally vaporize sections of the root, leaving a series of
clear surface images, which could be combined with software to make a 3D
rendering of the interior and exterior of the sample.
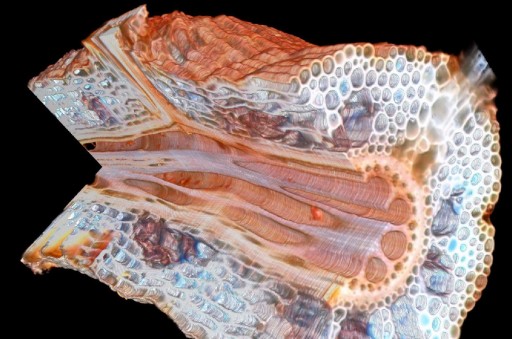
Maize
root with a cutaway showing a lateral root interface from Penn State’s
Roots lab (credit: Benjamin Hall/Penn State Materials Research
Institute)
The laser tomography method** is novel in that it provides high contrast, full color images without the use of contrast enhancing agents.
This allows researchers to see nuanced compositional differences in their samples they would not be able to see otherwise.
Additional benefits of the laser tomography method are its speed, on the order of minutes, and that in most cases no preparation is required for the small biological specimens studied.
[+]
Penn State has applied for a patent, and Hall and his business
partner Brian Reinhardt, a former Penn State graduate student, have
founded a company, Lasers for Innovative Solutions
(L4IS), aimed at providing large agriculture companies with high
throughput phenotyping of their new products, something they don’t
currently have.
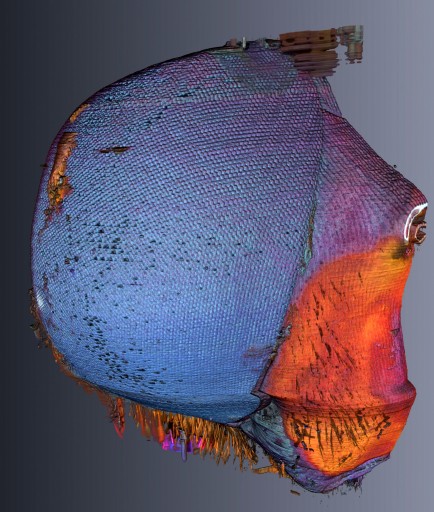
Bee fly (credit: Benjamin Hall/Penn State Materials Research Institute)
Ben Franklin Technology Partners of Central and Northern Pennsylvania provided funding and business assistance to start the company, and the Ben Franklin TechCelerator @ State College provided valuable entrepreneurial training.
Applications
“This technology is currently being applied to biological specimens, as it stands out as a promising solution for obtaining information about anatomical features rapidly for phenotyping,” Hall explained to KurzweilAI.
“We’ve done a range of specimens from tumor tissue, textiles, minerals, carbon nano-foam, and fungal-insect/plant interactions. Major Ag organizations are interested in understanding physiology/gene expression to drive product development. Additionally, we’ve explored applications in using the technique for analyzing shale samples for the oil and gas industry, to quantify vitrinite composition, total organic content, porosity, permeability, etc.
“A competitive technology which has been been the go-to method is Micro X-Ray tomography. This technology uses high energy x-rays to map out electron densities (roughly corresponds to material density) in specimens which can then be converted into a 3D rendering.
“However, that also means that materials with similar atomic composition but different molecular arrangements are difficult to resolve, requiring the use of heavy metal contrast enhancing agents. Since LAT uses light as a probe, nuanced differences in composition can be identified. For instance, as you’ll see in the whitepaper, it is possible to distinguish between the structural tissue with high lignin content from the high cellulose content in plant tissue.”
Might be useful for slicing small structures in brain tissue? I sent a note to Hall to find out. — Editor
Benjamin Hall replied:
“Size limits depend on material, but for biological tissue (like the brain) our current system could do somewhere around 20mm x 20mm x (unconstrained). We plan on trying tissue samples in the next couple of weeks to get a better idea of what kind of data we can get. I can keep you updated, if you like.”
* The standard method of preparing root samples for analysis requires cutting thin slices of root by hand, a process that yields four to five slices per hour. Jonathan Lynch, a professor of plant nutrition at Penn State and head of the Roots Lab, had a backlog of 20,000 samples he was studying to improve drought tolerance and nutritional uptake in low fertility soil.
Benjamin Hall was an undergraduate student in energy engineering working part-time in the laser lab of the Applied Research Laboratory at Penn State. Lynch applied for a small grant from the National Science Foundation’s Research Experience for Undergraduates (REU) program, which funded Hall to work on a project to apply lasers for slicing his root samples.
** “There are other [tomography techniques] out there,” says Hall. “But x-ray tomography basically works by mapping the density of a substance, which is great unless the specimen has different materials of similar density. That can make it hard to differentiate structures, so it can be difficult to quantify measurements. Magnetic resonance imaging (MRI) we’re not even competing with. Those machines are so big and complex, and so expensive to operate compared to our system.”
To view more awesome videos of their laser tomography: http://www.youtube.com/user/L4ISLLC
(¯`*• Global Source and/or more resources at http://goo.gl/zvSV7 │ www.Future-Observatory.blogspot.com and on LinkeIn Group's "Becoming Aware of the Futures" at http://goo.gl/8qKBbK │ @SciCzar │ Point of Contact: www.linkedin.com/in/AndresAgostini
 Washington
Washington